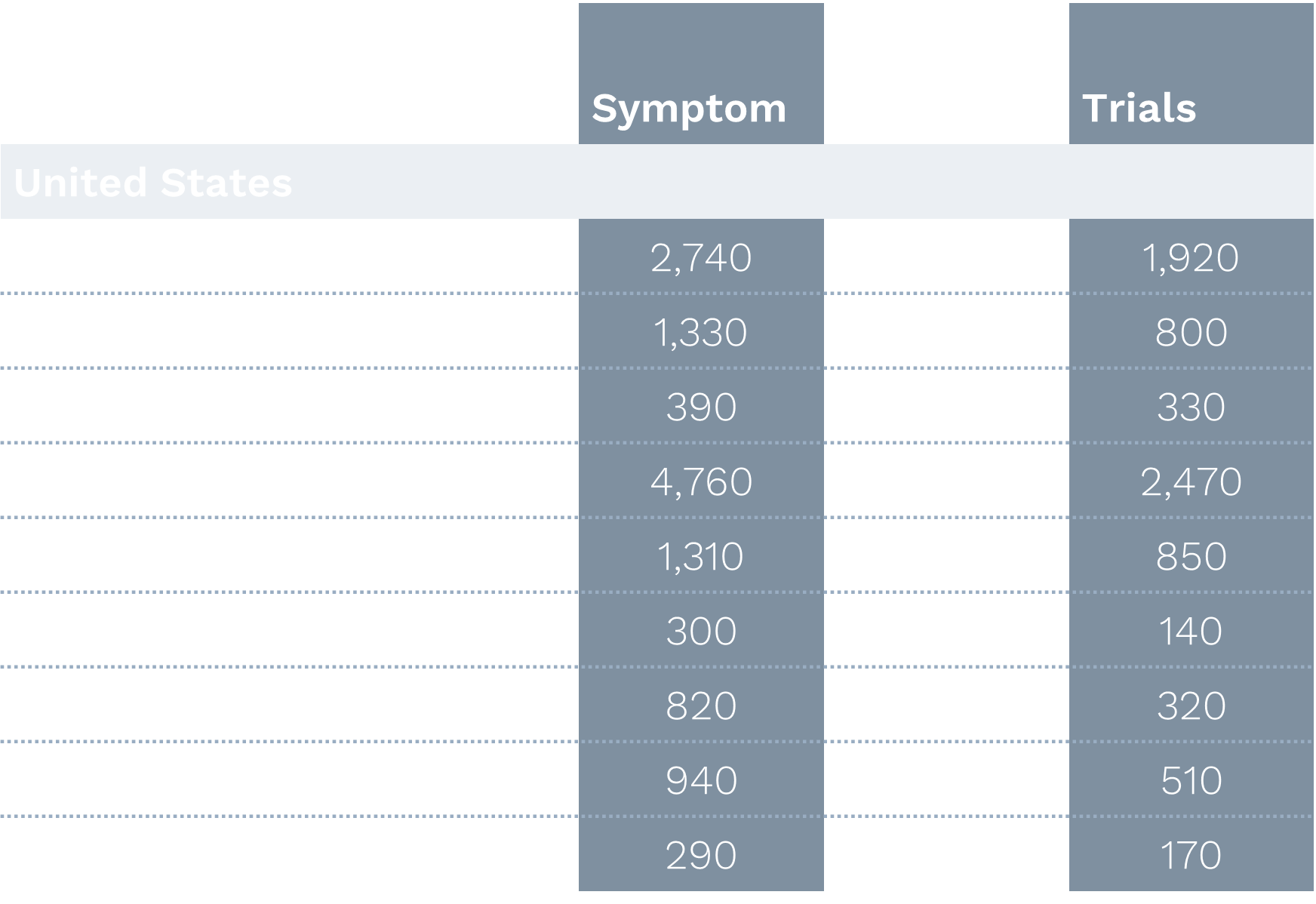
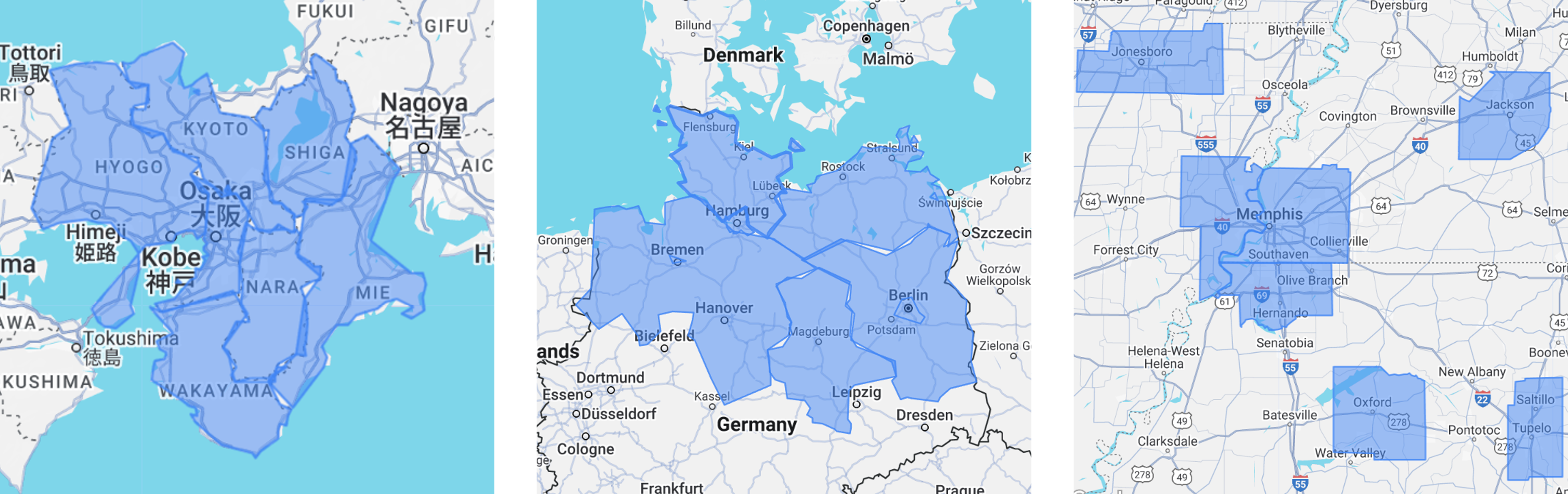
A comprehensive analysis of patient search behavior and digital engagement patterns for an Ulcerative Colitis (UC) trial reveals compelling insights into optimizing recruitment strategies across diverse global markets.
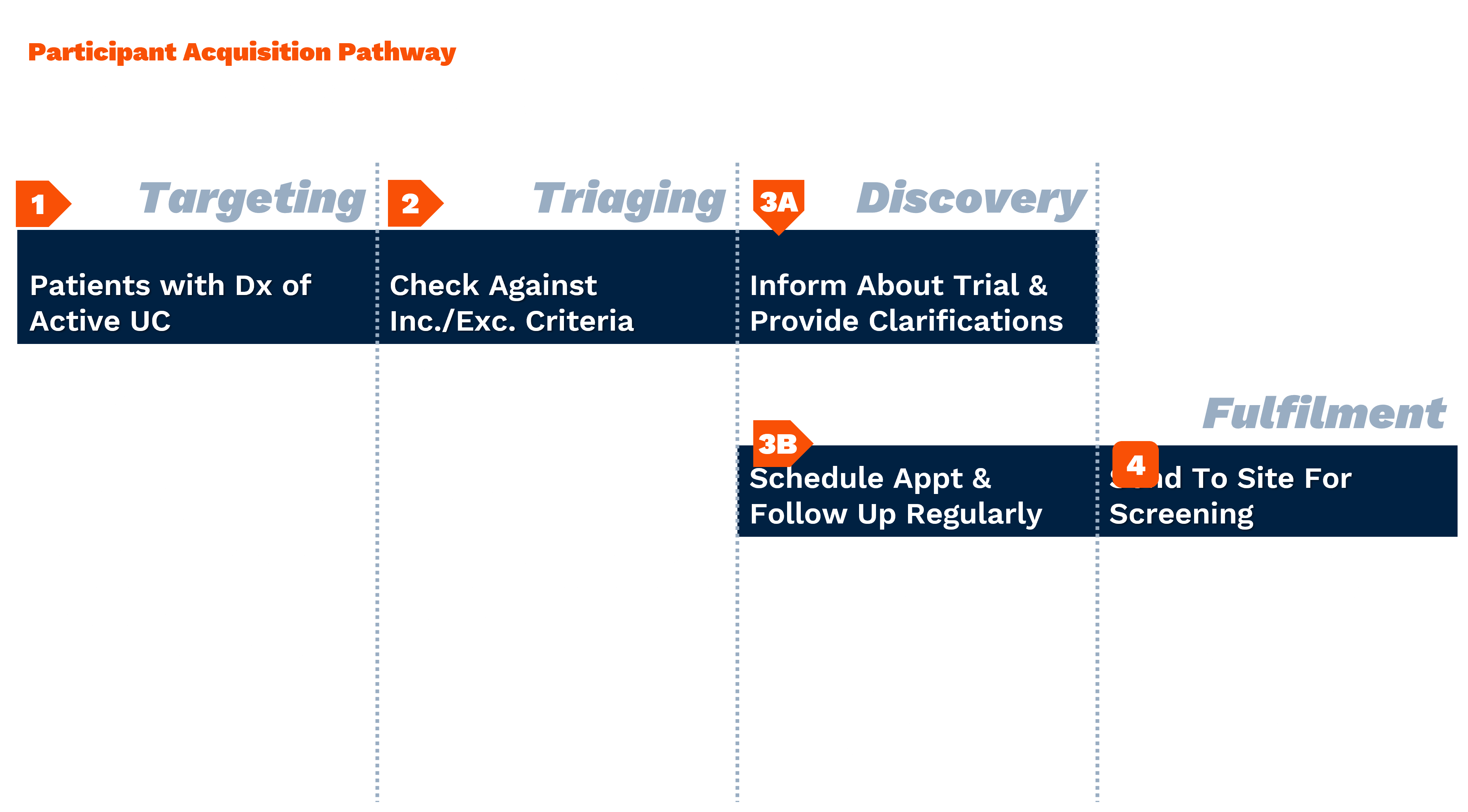
Methodology: The Curiosity Index
The analysis leveraged an innovative “Curiosity Index” examining three key dimensions:
- Symptom Curiosity: Patient research about UC symptoms like diarrhea and rectal bleeding
- Treatment Curiosity: Searches related to standard therapies including corticosteroids, immunosuppressants, and biologics
- Trial Curiosity: Interest in advanced treatments and clinical trials
Key Regional Insights
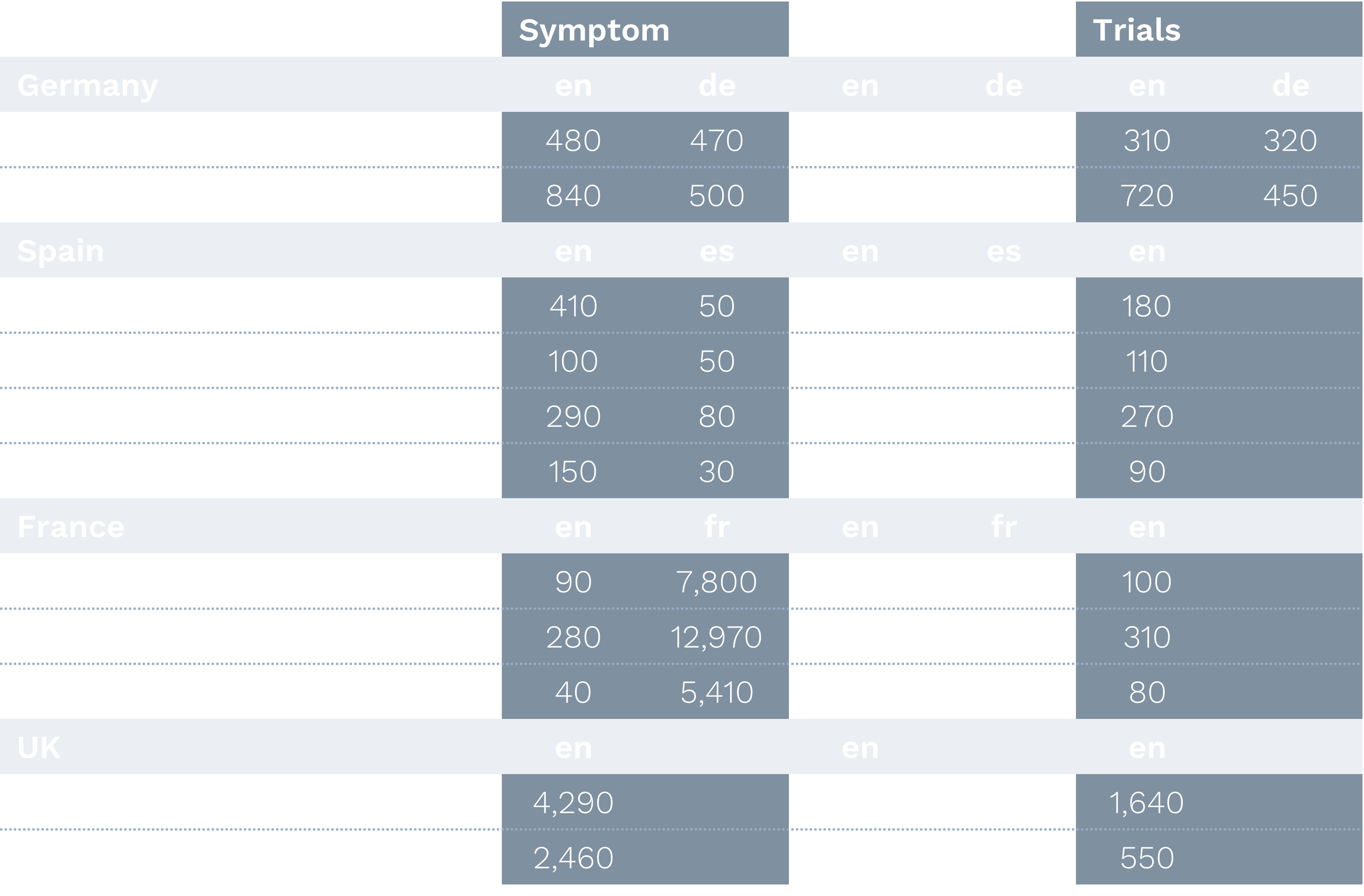
European Markets:
- Treatment curiosity significantly outweighed symptom curiosity across all regions
- French markets showed strong preference for native language content
- German markets displayed high bilingual comfort
- Secondary cities demonstrated significant engagement volumes
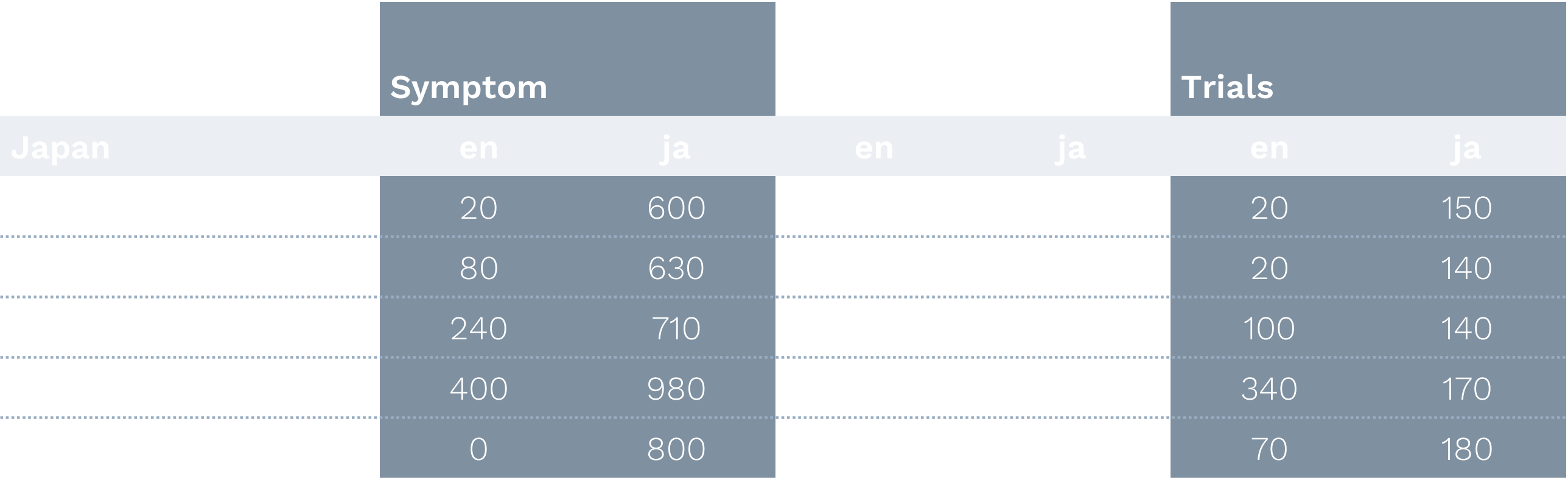
Japanese Markets:
- Extremely high treatment-to-symptom ratios
- Strong preference for Japanese language content
- Cities like Osaka and Saitama showed highest engagement
US Markets
- Higher trial curiosity compared to other regions
- Major metropolitan areas (Houston, Brooklyn) showed highest overall engagement
- Substantial engagement in secondary cities
Data-Driven Recommendations
Based on the analysis, key recommendations include:
- Content Strategy
- Design long-term nurture campaigns focused on treatment journey education
- Create culturally-specific trust-building content
- Implement targeted bilingual strategies
- Geographic Focus
- Prioritize metropolitan areas with high treatment curiosity
- Develop dedicated approaches for secondary cities
- Create educational pathways to convert treatment interest into trial participation
Performance Metrics
The platform demonstrates significant potential:
- 53% reduction in enrollment timelines
- 86% enrollments completed on time
- 62% referral-to-participation rate
This analysis demonstrates how data-driven insights can inform more effective, culturally sensitive recruitment strategies for Ulcerative Colitis trials.